Sd Biosensor Covid 19 Antigen Rapid Test Kit Sensitivity

Qualitatively detects specific antigens to sars cov 2 present in human nasopharynx.
Sd biosensor covid 19 antigen rapid test kit sensitivity. Sd biosensor lateral flow test. Date of phase 2 test. Covid 19 novel coronavirus testing kits are packaged on a production line at the sd biosensor bio diagnostic company near cheongju south korea. This product performs qualitative analysis by detecting influenza virus a type and b type antigens in the nasopharyngeal sample.
The organization will rank the tests based on sensitivity. This south korean brand donated 1 500 of its antigen test kits to cebu city in august. Authorities in spain and britain ordered millions of covid 19 test kits. Sd biosensor s covid 19 antigen rdt is granted who eul a first sd biosensor s covid 19 antigen rapid diagnostic test has been listed as eligible for who procurement and included under its emergency use listing on september 22 2020.
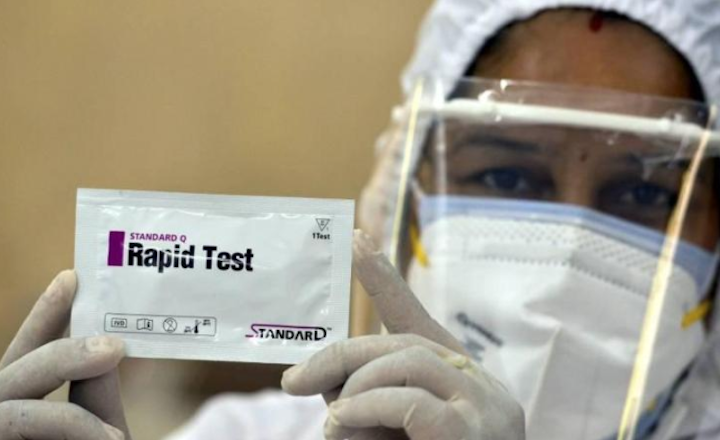
File photo on monday the indian council of medical research icmr approved one more kind of test for diagnosis of covid 19 the rapid antigen detection test is to be used in specified settings and kits from only one manufacturer have got approval. These kits are for professional use and are intended as a screening test to aid in the early diagnosis of sars cov 2 infection in patients with clinical symptoms. The test should be used as an aid in early diagnosis of symptomatic cases in healthcare setting having ili. Ed jones afp via getty images.
Standard q covid 19 igm igg duo test kit is a rapid immunochromatography test designed for the qualitative presumptive detection of specific igm and igg to sars cov 2 in humoral fluid. Rt pcr is currently the gold standard frontline test for the diagnosis of covid 19. 7 and 8 september 2020. This is the first eul for an antigen rdt.
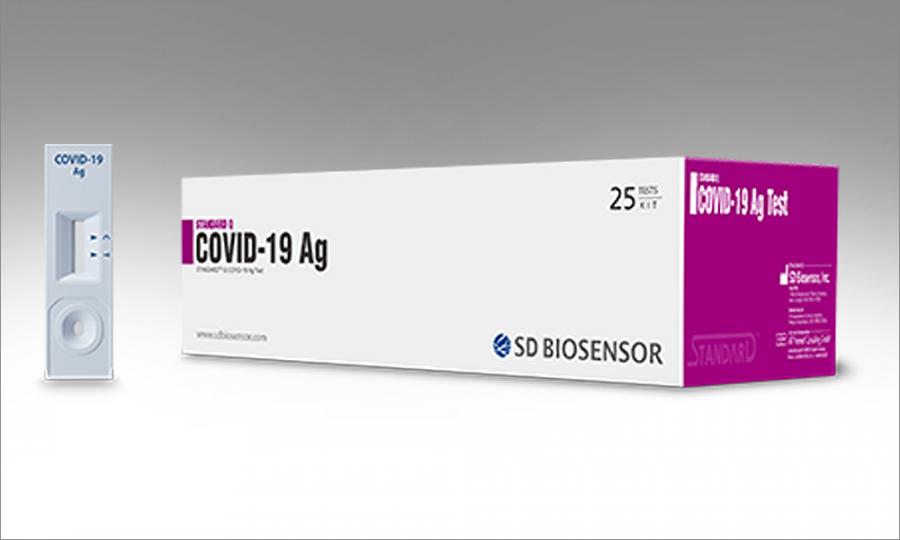
Sd biosensor s standard q covid 19 ag test was the first antigen test kit reviewed by the ritm. Coronavirus antigen rapid test. Standard q covid 19 ag is a rapid immunochromatographic assay for the qualitative detection of specific antigens to sars cov 2 present in human nasopharynx.